For the Equilibrium Br2 Cl2 2 Brcl at 400k
Thus P K p 4 4 1 4. Bromine chloride bromine and chlorine gas have reached equilibrium in a container at 500K according to the reaction 2BrCl Br2Cl2.
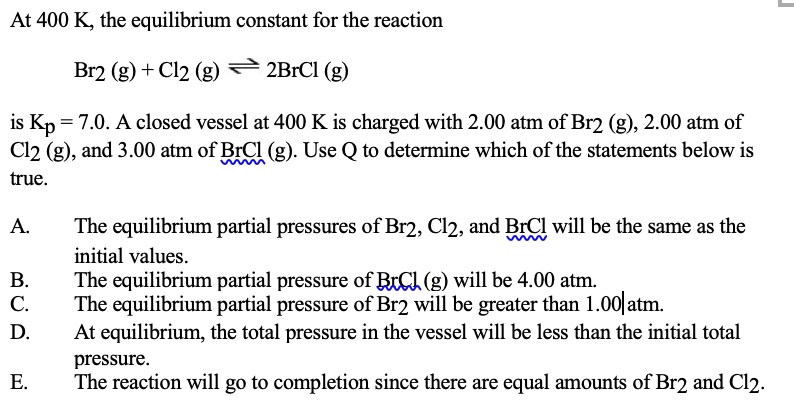
Solved At 400 K The Equilibrium Constant For The Reaction Br2 G Cl2 G 2brcl Is Kp 7 0 A Closed Vessel At 400 K Is Charged With 2 00 Atm Of Br2
This means that the enthalpy change for this reaction will be twice the value of ΔH f since.

. For the equilibrium below at 400K Kc70. Use Q to determine which of the statements is true below. 8 This reaction is proceeds in the gas phase or in the tetrachloromethane.
A closed vessel at 400K is charged with 100atm of Br2g 100atm of Cl2g and 200atm of BrClg. Brg C1g 2BrClg. Which of the statements below is true.
At 400 k the equilibrium constant for the reaction br2 g cl2 g 2brcl g is kp 70. For the equilibrium below at 400 K Kc 70. Use Q to determine which of the statements below is true The equilibrium partial pressures of Br2 Cl2 and BrCl will be the same as the initial values.
Br2 g Cl2 g 2BrClg features the formation of 2 moles of bromine monochloride. This problem needs the quadratic equation. For the equilibrium Br2g Cl2g 2BrCl g at 400 K Kf 70.
What will be the equilibrium concentration of Br Cl and BrCl. Equilibrium partial pressures are 108 bar for BrCl 49 bar Br2 and 76 bar Cl2. The equilibrium partial pressure of BrCl g will be greaterthan 200 atm.
Quiz 2 Problem 10. For the equilibrium below at 400 K Kc 70. The equilibrium partial pressures of Br2 Cl2 and BrCl will bethe same as the initial values.
At 400K the equilibrium constant for the reaction Br2gCl2g2BrClg is Kp70. Br2Cl22BrCl at 400K Kc70 if 025mol of Br2 and 055mol of Cl2 are introduced into a 30-l container at 400K what will be the equilibrium concentrations of In M A Br2 bCl2 C BrCl. I was working on the yellow book and I cant figure this question out.
At 400 K the equilibrium constant for the reaction Br2 g Cl2 g 2BrCl is Kp 70. At 400k the equilibrium constant for the reaction Br2g Cl2 g is Kp 70 p. If 00833M Br2 and 01833 M Cl2 are introduced into a container at 400K what will be the equilibrium concentrations of Br2 Cl2 and BrCl.
Br2gCl2g2BrClgKc70 Br 2 g Cl 2 g 2 BrCl g K c 70 If the composition of - 14101677. Suppose that 0500 mol IBr in a 200-L flask is allowed to reach equilibrium at 150 oC. Br2 g Cl2 g reverse reaction arrow 2 BrCl g If 090 mol of Br2 and 090 mol Cl2 are introduced into a 10 L container at 400.
Q17 Consider the reaction Clg 2C1g A flask is charged with 8 atm of pure CLg after which it is allowed to reach. The partial pressure of bromine is 9 P. A The equilbrium partial pressure of Br2 Cl2 and BrCl will be the same as the initial values B The equilibrium partial pressure of Br2 will be greater than 100 atm.
I know that Br2 and Cl2 have a 11 ratio and they each have a 12 ratio when compared to BrCl. 1 on a question. The equilibrium reaction is 2 N O B r g 2 N O g B r 2 g.
K what will be the equilibrium concentration of. Check the balance Bromine react with chlorine to produce bromine I chloride. If 025 mol of Br2 and 055 mol of Cl2 are introduces in a 3 L.
The equilibrium partial pressure of Br2 will be greater than100 atm. Click hereto get an answer to your question Q16 For the following equilibrium at 400K. Here ΔH rxn ΔH f.
For the equilibrium below at 400 K Kc 70. Br2g Cl2g 2 BrClg If 015 mol of Br2 and 015 mol Cl2 are introduced into a 10 L container at 400. For the reaction I2 Br2g Kc 280 at 150 For the reaction I2 Br2g Kc 280 at 150 oC.
Now the reaction given to you. At 400 k the equilibrium constant for the reaction br2 g cl2 g 2brcl g is kp 70. SIDE NOTE I was able to find a reference.
This reaction takes place at a temperature near 0C. Up to 256 cash back a. K what will be the equilibrium concentration of.
View EquilibriumKEY from CHM 116 at Arizona State University. Use q to determine which of the statements below is true. The partial pressures of N O B r and N O will be 9 7 P and 9 2 P respectively.
Moles of Br2 035 Moles of Cl2 05 Volume 3 L M of Br2 0116667. At equilibrium the total pressure in the vessel will be lessthan the initial total pressure. Br2g Cl2g 2BrClg If 039 mol of Br2 and 039 mol Cl2 are introduced into a 10L container at 400K what will be the equilibrium concentration of BrCl.
C-At equilibrium the total pressure in the vessel will be less than the initial total pressure. B-The equilibrium partial pressure of Br2 will be greater than 100 atm. A closed vessel at 400 K is charged with 200 atm of Br2 g 200 atm of Cl2 g and 300 atm of BrCL g.
A-The equilibrium partial pressure of BrCl g will be greater than 200 atm. Who are the experts. If 035 mol of Br2 and 050 mol of Cl2 are introduces in a 3 L container what will be the equilibrium concentration of each of the components.
Draw the ice table. Br 2 Cl 2 2BrCl. Science Chemistry QA Library For the equilibrium Br2g Cl2g -- -- 2 BrClg At 400K Kc70.
C The equilibrium partial pressure of BrCl g will be greater than 200 atm D The reaction will go to completion since there are equal amounts of Br2 and Cl2. The expression for the equilibrium constant will be K p P N O B r 2 P N O 2 P B r 2 9 7 P 2 9 2 P 2 9 P. Br2g Cl2g 2 BrCL 9 Each of the three gases is introduces at a concentration of 20 molL in a container.
Consider this equilibrium reaction at 400 K. A closed vessel at 400k is charged with 1 atm of Br2g 1 atm of Cl2 g and 200 atm of BrClg. D-The reaction will go to completion since there are equal amounts of Br2 and Cl2.
Experts are tested by Chegg as specialists in their subject area. Question 10 5 points For the reaction given below the value of the equilibrium constant at 400K is 70. 2moles BrCl ΔH f 1mole BrCl 2 ΔH f.
For the equilibrium Br2g Cl2g 2BrCl g at 400K Kf 70. Br2g Cl2g reverse reaction arrow 2 BrClg If 090 mol of Br2 and 090 mol Cl2 are introduced into a 10 L container at 400. Kc 9 If 5 mol of Br and 5 mol of Cl are introduced in 5L container at 400 K.
A The partial pressures represent a system in equilibrium b The reaction will proceed to the left c The. A closed vessel at 400 k is charged with 100 atm of br2 g 100 atm of cl2 g and 200 atm of brcl g. This problem has been solved.

For The Equilibrium Br2 G Cl2 G 2brcl G At 400 K Kc 7 0 Part A If 0 25 Mol Of Homeworklib

Solved At 400 K The Equilibrium Constant For The Reaction Chegg Com
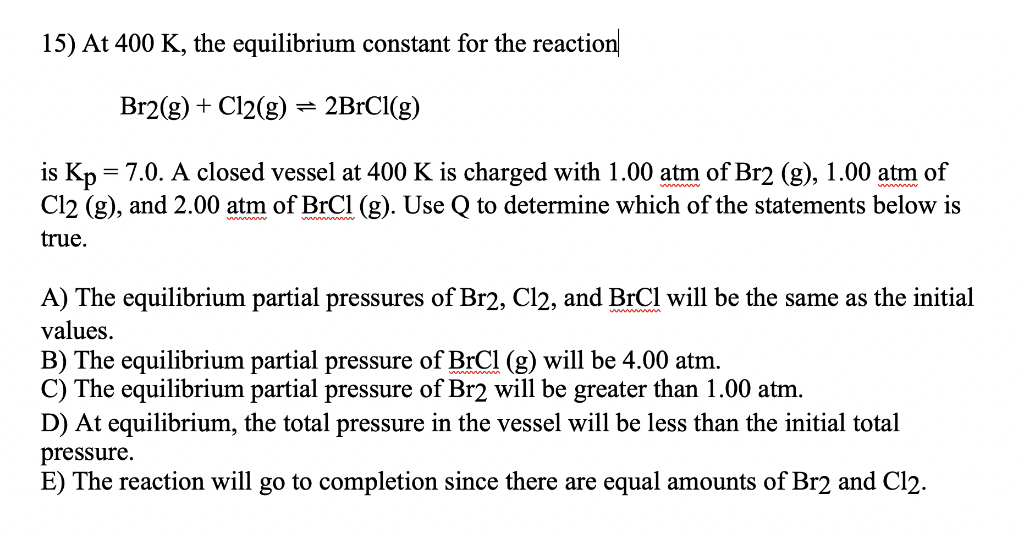
Solved 15 At 400 K The Equilibrium Constant For The Chegg Com
Comments
Post a Comment